Glutathione (GSH) is synthesized in the cytoplasm in virtually all cells from its constituent amino acids by two sequential ATP-requiring enzyme-catalyzed reactions (see figure below) [1]. :

The first reaction is rate-limiting and is catalysed by glutamate cysteine ligase (GCL, EC 6.3.2.2; formerly known as γ-glutamylcysteine synthetase). GCL is composed of a heavy catalytic subunit (GCLC, Mwt ~ 73,000) and a light modifier (GCLM, Mwt ~ 30,000) subunit. GCL is the key control point for the homeostasis of cellular GSH and is regulated at multiple levels. Its regulation both at a genetic level (both transcriptional and translational) and a biochemical level (post-translational) is incredibly complex. As to be expected with any such complex regulation, the likelihood of errors that result in the impairment of function are increased. The cause and effect of errors in the control mechanisms of GCL are slowly being unravelled by researchers and is the subject of several excellent reviews [2-6]. A dysfunctional GCL will result in insufficient γ-glutamylcysteine (Glyteine) being produced for the glutathione synthase enzyme to convert into GSH. The resulting suboptimal homeostasis of GSH results in a decreased capacity to minimize oxidative stress, which is associated with poor outcomes during ageing and in multiple disease states. This is the theoretical basis for endogenously supplied gamma-glutamylcysteine’s ability to raise GSH levels, as being the product of the GCL enzyme, it can be taken up by cells intact, where it feeds directly into glutathione synthase to be converted to glutathione, effectively bypassing the dysfunctional GCL.
The light modifier subunit GCLM is enzymatically inactive but plays an important regulatory function by lowering the Km of GCLC for glutamate and raising the Ki for GSH [7, 8]. The resulting holoenzyme dimer comprising the two subunits is catalytically more efficient and less subject to inhibition by GSH than GCLC alone. In rats, GCLC has a Km for glutamate that is about 10-fold higher, which is higher than the cellular glutamate concentration in most tissues.
GCL is specific for the glutamyl moiety and is regulated physiologically by: (a) non-allosteric feedback competitive inhibition by GSH (Ki=2.3mM), which involves binding of GSH to the glutamate and another site on the enzyme [8, 9] and (b) availability of its precursor, cysteine [1].The apparent Km values of GCL for glutamate is 1.8 mM, and for cysteine and 0.1–0.3 mM [9]. The intracellular glutamate concentration is nearly 10-fold higher than the Km value, but intracellular cysteine concentrations approximate the apparent Km value [10], indicating that cysteine may be a limiting substrate for GCL. This has led to the false assumption that the cause of GSH depletion associated with so many chronic diseases must be from an under supply of cysteine in the diet. This is an unlikely scenario as the developed world’s dietary intake of sulfur amino acids is generally adequate. For example, the typical American diet supplies much more than the recommended required quantity of cysteine [11]. Interestingly, this has not discouraged multiple research groups testing cysteine prodrugs, such as N-acetylcysteine (NAC), as supplements to increase GSH levels. Unsurprisingly, most clinical trials with NAC have resulted in lack of therapeutic efficacy [12-16]. However, NAC has been shown to restore GSH levels in cases such as acetaminophen (paracetamol) overdose, where an acute depletion of GSH is observed [17]. In this case, GSH levels are well below the normal concentration, and therefore there is no feedback inhibition of GCL. To date, the only medically recognized use of NAC is in acetaminophen poisoning.
Our proposition is that suboptimal GSH levels associated with so many chronic diseases is not due to a cysteine undersupply but an impairment in one of the multiple control mechanisms of the GCL enzyme. Again, this impairment is effectively bypassed by supplementation with gamma-glutamylcysteine, which can be transported into cells where it will be rapidly converted to GSH by GSH synthase.
The second step in GSH synthesis is catalyzed by GSH synthase (GS, EC 6.3.2.3, formerly known as GSH synthetase). GS is constitutively expressed in all cells and has not been studied extensively as GCL [5]. It is not subject to feedback inhibition by GSH, and its substrates gamma-glutamylcysteine and glycine are rapidly ligated to form GSH. The intracellular concentration of gamma-glutamylcysteine is extremely low when GS is present; thus, GCL is considered the rate-limiting enzyme [6]. In support of this observation, over expression of GS in a yeast model failed to increase GSH levels, whereas overexpression of GCL did [18].
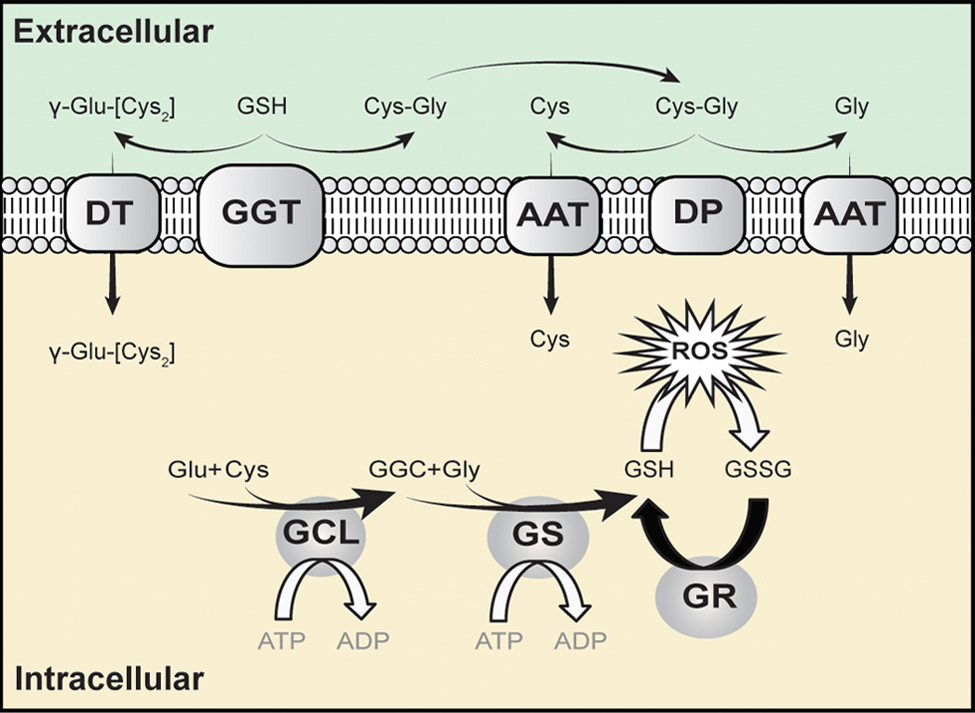
The intracellular concentration of GSH is in the millimolar range, and the extracellular concentration is in the micromolar range. This steep gradient from inside the cell to outside the cell drives the export of GSH. This concentration gradient makes transport of GSH from outside the cell to inside the cell thermodynamically unfavourable. It is not surprising then that attempts to increase intracellular GSH by supplementation with GSH have been unsuccessful [19, 20]. Once outside the cell, GSH degradation occurs exclusively in the extracellular space and only on the surface of cells that express the ectoenzyme γ-glutamyl transferase. This enzyme catalyses the transfer of the γ-glutamyl moiety of GSH to another amino acid to produce a γ-Glu-AA and cysteinyl-glycine. These γ-Glu-AAs can be transported back into cells, where they become substrates for the enzyme γ-glutamyl cyclotransferase. This enzyme generates 5-oxoproline and releases the amino acid or peptide that is bound to glutamate. 5-Oxoproline is converted to glutamate by the ATP-requiring enzyme 5-oxoprolinase. The other part of the hydrolysed GSH molecule cysteinyl-glycine is transported back inside the cell and broken down further by nonspecific dipeptidases into cysteine and glycine, ready for resynthesis back into GSH. This process allows for the release of cysteine from GSH, which can then be used in protein synthesis. In this case, GSH is functioning as a storage mechanism for cysteine which is otherwise unstable in its free form.
GSH is a cofactor, coenzyme, and/or substrate for a number of enzymes and can participate in a number of redox and conjugation reactions. Within the cell, it exists mainly (98%) in the thiol-reduced form (GSH), but some are also present as glutathione disulfide (GSSG). This ratio of GSH: GSSG is maintained by the action of GSSG reductase, which requires NADPH+ as the reducing cofactor. GSH can react with many electrophilic compounds to generate glutathione S-conjugates. Free radicals and other oxidants are reduced by the direct action of GSH and indirectly via enzyme glutathione peroxidase, which use GSH as a cofactor.
References
- Meister, A. and M.E. Anderson, Glutathione. Annu Rev Biochem, 1983. 52: p. 711-60.
- Ferguson, G. and W. Bridge, Glutamate cysteine ligase and the age-related decline in cellular glutathione: The therapeutic potential of γ-glutamylcysteine. Archives of Biochemistry and Biophysics, 2016. 593: p. 12-23.
- Franklin, C.C., et al., Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Molecular Aspects of Medicine, 2009. 30(1-2): p. 86-98.
- Ballatori, N., et al., Glutathione dysregulation and the etiology and progression of human diseases. Biological Chemistry, 2009. 390(3): p. 191-214.
- Lu, S.C., Glutathione synthesis. Biochimica et Biophysica Acta (BBA) – General Subjects, 2013. 1830(5): p. 3143-3153.
- Dalton, T.P., et al., Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radical Biology and Medicine, 2004. 37(10): p. 1511-1526.
- Huang, C.S., M.E. Anderson, and A. Meister, Amino acid sequence and function of the light subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem, 1993. 268(27): p. 20578-83.
- Huang, C.S., et al., Catalytic and regulatory properties of the heavy subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem, 1993. 268(26): p. 19675-80.
- Richman, P.G. and A. Meister, Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem, 1975. 250(4): p. 1422-6.
- Bannai, S. and N. Tateishi, Role of membrane transport in metabolism and function of glutathione in mammals. J Membr Biol, 1986. 89(1): p. 1-8.
- Lang, C.A., The impact of glutathione on health and longevity. Journal of Anti Aging Medicine, 2001. 4(2): p. 137-144.
- Aitio, M.-L., N-acetylcysteine – passe-partout or much ado about nothing? British Journal of Clinical Pharmacology, 2006. 61(1): p. 5-15.
- Ashworth, A. and S.T. Webb, Does the prophylactic administration of N-acetylcysteine prevent acute kidney injury following cardiac surgery? Interact CardioVasc Thorac Surg, 2010. 11(3): p. 303-308.
- Sochman, J., N-Acetylcysteine Somewhere Between Scylla and Charybdis. J Am Coll Cardiol, 2010. 56(13): p. 1067-a-.
- Wang, G., et al., N-acetylcysteine in Cardiac Surgery: Do the Benefits Outweigh the Risks? A Meta-Analytic Reappraisal. Journal of cardiothoracic and vascular anesthesia, 2011. 25(2): p. 268-275.
- Coles, L.D., et al., Repeated-Dose Oral N-Acetylcysteine in Parkinson’s Disease: Pharmacokinetics and Effect on Brain Glutathione and Oxidative Stress. The Journal of Clinical Pharmacology, 2018. 58(2): p. 158-167.
- Rushworth, G.F. and I.L. Megson, Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacology & Therapeutics, 2014. 141(2): p. 150-159.
- Grant, C.M., F.H. MacIver, and I.W. Dawes, Glutathione synthetase is dispensable for growth under both normal and oxidative stress conditions in the yeast Saccharomyces cerevisiae due to an accumulation of the dipeptide gamma-glutamylcysteine. Mol Biol Cell, 1997. 8(9): p. 1699-707.
- Witschi, A., et al., The systemic availability of oral glutathione. European Journal of Clinical Pharmacology, 1992. 43(6): p. 667-669.
- Allen, J. and R.D. Bradley, Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J Altern Complement Med, 2011. 17(9): p. 827-33.
- Amir Aslani, B. and S. Ghobadi, Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci, 2016. 146: p. 163-73.
- Morris, D., et al., Glutathione and infection. Biochimica et Biophysica Acta (BBA) – General Subjects, 2013. 1830(5): p. 3329-3349.
- Ghezzi, P., Role of glutathione in immunity and inflammation in the lung. International Journal of General Medicine, 2011. 4: p. 105-113.
- Perricone, C., C. De Carolis, and R. Perricone, Glutathione: A key player in autoimmunity. Autoimmunity Reviews, 2009. 8(8): p. 697.
- Rodrigues, C. and S.S. Percival, Immunomodulatory Effects of Glutathione, Garlic Derivatives, and Hydrogen Sulfide. Nutrients, 2019. 11(2).
- Biswas, S.K. and I. Rahman, Environmental toxicity, redox signaling and lung inflammation: The role of glutathione Molecular Aspects of Medicine, 2009. 30(1-2): p. 60.
- Yuan, L. and N. Kaplowitz, Glutathione in liver diseases and hepatotoxicity. Molecular Aspects of Medicine, 2009. 30: p. 29.
- Guilford, F.T. and J. Hope, Deficient glutathione in the pathophysiology of mycotoxin-related illness. Toxins (Basel), 2014. 6(2): p. 608-23.
- Dringen, R., et al., Glutathione-Dependent Detoxification Processes in Astrocytes. Neurochem Res, 2015. 40(12): p. 2570-82.
- Bocedi, A., et al., Glutathione Transferase P1-1 an Enzyme Useful in Biomedicine and as Biomarker in Clinical Practice and in Environmental Pollution. Nutrients, 2019. 11(8).
- Morris, G., et al., The Glutathione System: A New Drug Target in Neuroimmune Disorders. Molecular Neurobiology, 2014: p. 1-26.
- Aoyama, K. and T. Nakaki, Impaired Glutathione Synthesis in Neurodegeneration. International Journal of Molecular Sciences, 2013. 14(10): p. 21021-21044.
- Johnson, W.M., A.L. Wilson-Delfosse, and J.J. Mieyal, Dysregulation of Glutathione Homeostasis in Neurodegenerative Diseases. Nutrients, 2012. 4(10): p. 1399-1440.
- Cacciatore, I., et al., Recent advances in the treatment of neurodegenerative diseases based on GSH delivery systems. Oxidative Medicine and Cellular Longevity, 2012.
- McBean, G.J., et al., Thiol redox homeostasis in neurodegenerative disease. Redox Biol, 2015. 5: p. 186-194.
- Gu, F., V. Chauhan, and A. Chauhan, Glutathione redox imbalance in brain disorders. Curr Opin Clin Nutr Metab Care, 2015. 18(1): p. 89-95.
- Rae, C.D. and S.R. Williams, Glutathione in the human brain: Review of its roles and measurement by magnetic resonance spectroscopy. Anal Biochem, 2017. 529: p. 127-143.
- Cao, P., et al., Therapeutic approaches to modulating glutathione levels as a pharmacological strategy in Alzheimer’s disease. Curr Alzheimer Res, 2015. 12(4): p. 298-313.
- Kettle, A.J., et al., Oxidation contributes to low glutathione in the airways of children with cystic fibrosis. Eur Respir J, 2014. 44(1): p. 122-9.
- Fitzpatrick, A.M., D.P. Jones, and L.A.S. Brown, Glutathione Redox Control of Asthma: From Molecular Mechanisms to Therapeutic Opportunities. Antioxidants & Redox Signaling, 2012. 17(2): p. 375-408.
- Reynaert, N.L., Glutathione biochemistry in asthma Biochimica et Biophysica Acta (BBA) – General Subjects, 2011. In Press, Corrected Proof.
- Gould, N.S. and B.J. Day, Targeting maladaptive glutathione responses in lung disease. Biochemical Pharmacology, 2011. 81(2): p. 187-193.
- Circu, M.L. and T.Y. Aw, Redox biology of the intestine. Free Radical Research, 2011. 45(11-12): p. 1245-1266.
- Desideri, E., F. Ciccarone, and M.R. Ciriolo, Targeting Glutathione Metabolism: Partner in Crime in Anticancer Therapy. Nutrients, 2019. 11(8).
- Bansal, A. and M.C. Simon, Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol, 2018. 217(7): p. 2291-2298.
- Hatem, E., N. El Banna, and M.E. Huang, Multifaceted Roles of Glutathione and Glutathione-Based Systems in Carcinogenesis and Anticancer Drug Resistance. Antioxid Redox Signal, 2017. 27(15): p. 1217-1234.
- Traverso, N., et al., Role of Glutathione in Cancer Progression and Chemoresistance. Oxidative Medicine and Cellular Longevity, 2013. 2013: p. 10.
- Reuter, S., et al., Oxidative stress, inflammation, and cancer: how are they linked? Free radical biology & medicine, 2010. 49(11): p. 1603-1616.
- Bajic, V.P., et al., Glutathione “Redox Homeostasis” and Its Relation to Cardiovascular Disease. Oxid Med Cell Longev, 2019. 2019: p. 5028181.
- Kanaan, G.N. and M.E. Harper, Cellular redox dysfunction in the development of cardiovascular diseases. Biochim Biophys Acta Gen Subj, 2017. 1861(11 Pt A): p. 2822-2829.
- Ndrepepa, G. and A. Kastrati, Gamma-glutamyl transferase and cardiovascular disease. Ann Transl Med, 2016. 4(24): p. 481.
- Mistry, R.K. and A.C. Brewer, Redox-Dependent Regulation of Sulfur Metabolism in Biomolecules: Implications for Cardiovascular Health. Antioxid Redox Signal, 2019. 30(7): p. 972-991.
- Mulcahy, R.T., et al., Constitutive and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/TRE sequence. Journal of Biological Chemistry, 1997. 272(11): p. 7445-7454.
- Mulcahy, R.T. and J.J. Gipp, Identification of a putative antioxidant response element in the 5′-flanking region of the human gamma-glutamylcysteine synthetase heavy subunit gene. Biochem Biophys Res Commun, 1995. 209(1): p. 227-33.
- Galloway, D.C., et al., Regulation of human gamma-glutamylcysteine synthetase: co-ordinate induction of the catalytic and regulatory subunits in HepG2 cells. Biochem J, 1997. 328 ( Pt 1): p. 99-104.
- Moinova, H.R. and R.T. Mulcahy, An electrophile responsive element (EpRE) regulates beta-naphthoflavone induction of the human gamma-glutamylcysteine synthetase regulatory subunit gene. Constitutive expression is mediated by an adjacent AP-1 site. J Biol Chem, 1998. 273(24): p. 14683-9.
- Lee, T.D., et al., Cloning and characterization of the human glutathione synthetase 5′-flanking region. Biochem J, 2005. 390(Pt 2): p. 521-8.
- Wild, A.C., H.R. Moinova, and R.T. Mulcahy, Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem, 1999. 274(47): p. 33627-36.
- Moinova, H.R. and R.T. Mulcahy, Up-regulation of the human gamma-glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf-2 to an electrophile responsive element. Biochem Biophys Res Commun, 1999. 261(3): p. 661-8.
- Yang, H., et al., Nrf1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-kappaB and AP-1. Mol Cell Biol, 2005. 25(14): p. 5933-46.
- Wild, A.C. and R.T. Mulcahy, Regulation of gamma-glutamylcysteine synthetase subunit gene expression: Insights into transcriptional control of antioxidant defenses. Free Radical Research, 2000. 32(4): p. 281-301.
- Dickinson, D.A., et al., Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radical Biology and Medicine, 2004. 37(8): p. 1152-1159.
- Cai, J., W.M. Sun, and S.C. Lu, Hormonal and cell density regulation of hepatic gamma-glutamylcysteine synthetase gene expression. Mol Pharmacol, 1995. 48(2): p. 212-8.
- Lu, S.C., et al., Insulin and Glucocorticoid Dependence of Hepatic Gamma-Glutamylcysteine Synthetase and Glutathione Synthesis in the Rat – Studies in Cultured-Hepatocytes and Invivo. Journal of Clinical Investigation, 1992. 90(2): p. 524-532.
- Cai, J., Z.Z. Huang, and S.C. Lu, Differential regulation of gamma-glutamylcysteine synthetase heavy and light subunit gene expression. Biochem J, 1997. 326 ( Pt 1): p. 167-72.
- Rahman, I., et al., Characterisation of gamma-glutamylcysteine synthetase-heavy subunit promoter: a critical role for AP-1. Febs Letters, 1998. 427(1): p. 129-133.
- Lu, S.C., Glutathione synthesis. Biochim Biophys Acta, 2013. 1830(5): p. 3143-53.
- Kaspar, J.W., S.K. Niture, and A.K. Jaiswal, Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med, 2009. 47(9): p. 1304-9.
- Erickson, A.M., et al., Identification of a variant antioxidant response element in the promoter of the human glutamate-cysteine ligase modifier subunit gene. Revision of the ARE consensus sequence. J Biol Chem, 2002. 277(34): p. 30730-7.
- Kwong, M., Y.W. Kan, and J.Y. Chan, The CNC basic leucine zipper factor, Nrf1, is essential for cell survival in response to oxidative stress-inducing agents. Role for Nrf1 in gamma-gcs(l) and gss expression in mouse fibroblasts. J Biol Chem, 1999. 274(52): p. 37491-8.
- Yang, H., et al., Tumour necrosis factor alpha induces co-ordinated activation of rat GSH synthetic enzymes via nuclear factor kappaB and activator protein-1. Biochem J, 2005. 391(Pt 2): p. 399-408.
- Rushworth, S.A., S. Shah, and D.J. MacEwan, TNF mediates the sustained activation of Nrf2 in human monocytes. J Immunol, 2011. 187(2): p. 702-7.
- Benassi, B., et al., c-Myc phosphorylation is required for cellular response to oxidative stress. Molecular Cell, 2006. 21(4): p. 509-519.
